
MicroVention Announces First U.S. Patient Treated with FRED™ X™ Flow Diverter Featuring X Technology
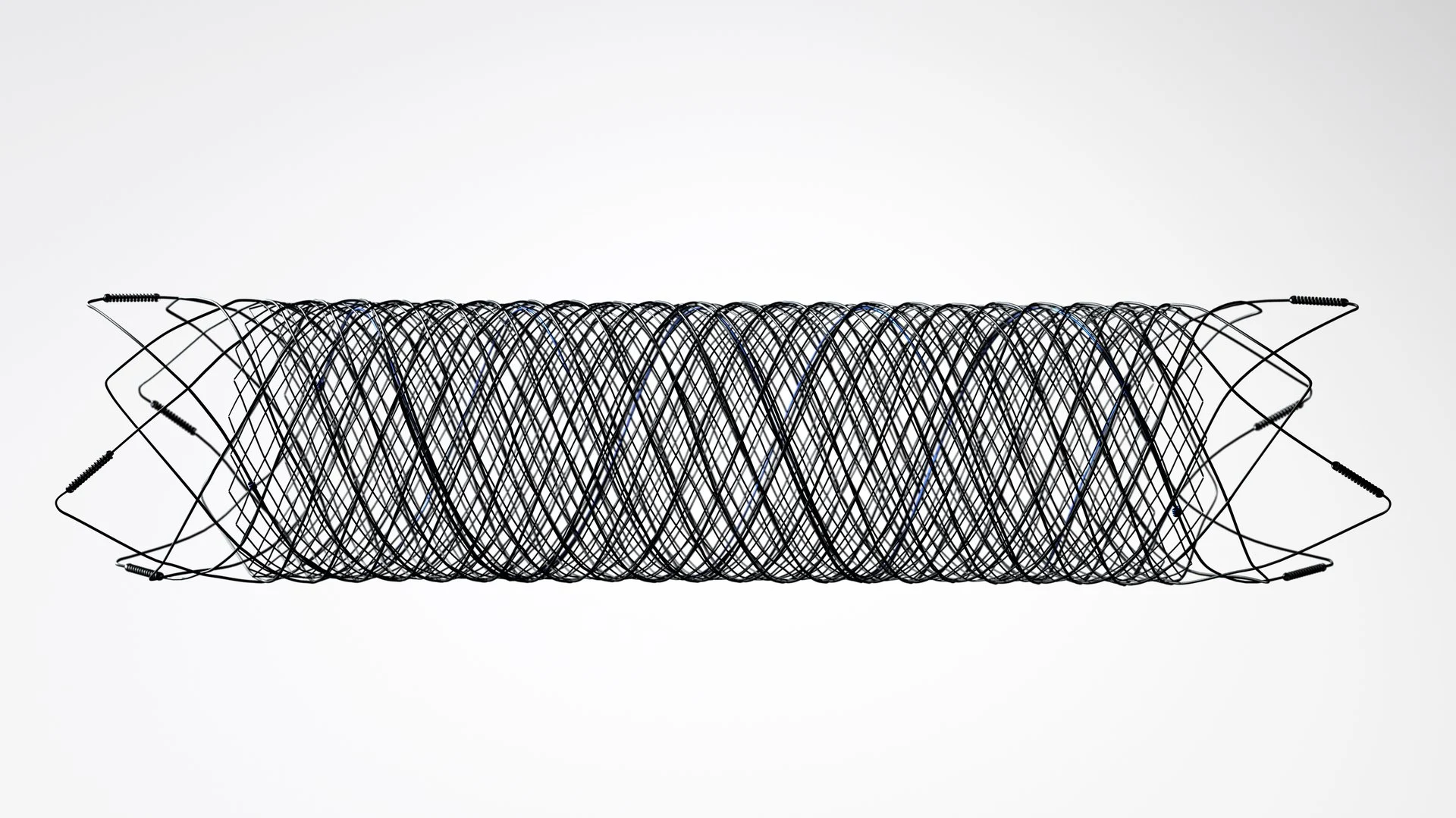
Aliso Viejo, CA. – February 22, 2022 – MicroVention, Inc., a subsidiary of Terumo and a global neurovascular company, announced the first U.S. clinical case of its next generation Flow Diverter, the FRED X device, at Thomas Jefferson University Hospital located in Philadelphia. MicroVention received FDA PMA approval for the FRED X device in September of last year. The FRED X Flow Diverter features MicroVention X Technology, a proprietary nano-polymer surface modification designed to reduce material thrombogenicity, or tendency for clot formation on the device surface.
Building on the original FRED device, which received FDA PMA approval in 2019, the FRED X device uses a self-expanding braided nitinol mesh to help re-direct blood flow and promote aneurysm occlusion. With the addition of X Technology, FRED X is designed to reduce thrombogenicity of the device material and enhance blood vessel healing. FRED X offers both the smallest and largest flow diversion systems available in the U.S., providing physicians with the broadest range of options for treating their patients.
“FRED X represents an important advancement in flow diversion therapy. The X Technology surface treatment applied to the FRED X Device is designed to reduce material thrombogenicity and has further increased the deliverability of the FRED X,” said Dr. Stavropoula Tjoumakaris, Director, Endovascular Surgery and Cerebrovascular Neurosurgery Fellowship at Thomas Jefferson University Hospital, and principal investigator of the FRED X [post approval] study. “We are excited to be the first center in the U.S. to have clinical experience using the FRED X device and look forward to a clinical study on the benefits of X Technology.”
An estimated 6.5 million people [or 1 in 50 people] in the U.S. have an unruptured brain aneurysm, approximately 300,000 of which will rupture, potentially leading to disability or death. Since it received the CE Mark in 2013, the FRED device has been used to treat tens of thousands of patients and its safety and effectiveness have been shown in dozens of clinical publications.
“At MicroVention, we continue to enhance our product portfolio to make meaningful improvements in patient care,” said Carsten Schroeder, President and CEO of MicroVention. “Surface treatment technologies, such as the FRED X device, represent the next frontier in neurovascular therapy, and MicroVention is proud to be able to offer the first of several devices incorporating our X Technology to physicians and patients in the U.S.”
For more information regarding the FRED X Flow Diversion Device, please visit MicroVention’s website at www.microvention.com.
About the FRED X Device
The FRED X device is indicated for use in the internal carotid artery from the petrous segment
to the terminus for the endovascular treatment of adult patients (22 years of age or older) with
wide-necked (neck width ≥ 4 mm or dome-to-neck ratio < 2) saccular or fusiform intracranial
aneurysms arising from a parent vessel with a diameter ≥ 2.0 mm and ≤ 5.0 mm.
About MicroVention, Inc.
Founded in 1997, MicroVention develops and markets medical devices that enable or
significantly improve treatment of cerebrovascular diseases. In 2006, Terumo Corporation, a
major worldwide medical device company headquartered in Tokyo, Japan, acquired
MicroVention into its family of Companies. Terumo’s acquisition of MicroVention allowed both
Companies to leverage their unique, proprietary technologies toward an increased focus on
treating cerebrovascular diseases. Headquartered in California, MicroVention products are sold
in more than 75 nations through a direct sales organization alongside strategic distribution
partnerships.
www.microvention.com
About Terumo
Terumo (TSE: 4543) is a global leader in medical technology and has been committed to
“Contributing to Society through Healthcare” for 100 years. Based in Tokyo and operating
globally, Terumo employs more than 25,000 associates worldwide to provide innovative
medical solutions in more than 160 countries and regions. The company started as a Japanese
thermometer manufacturer and has been supporting healthcare ever since. Now its extensive
business portfolio ranges from vascular intervention and cardio-surgical solutions, blood
transfusion and cell therapy technology, to medical products essential for daily clinical practice,
such as transfusion systems, diabetes care, and peritoneal dialysis treatments. Terumo will
further strive to be of value to patients, medical professionals, and society at large.
www.terumo.com
Resources
MicroVention Announces First Patient Enrolled in the STRAIT Study with the New BOBBY™ Balloon Guide Catheter
Apr 20, 2022
MicroVention, Inc., a wholly owned subsidiary of Terumo Corporation, announced the successful completion of its first enrollment in a multi-center, prospective EU observational study called STRAIT, to evaluate the Safety and Performance of the BOBBY Balloon Guide Catheter for Endovascular Treatment of Acute Ischemic Stroke.
Lower-Profile WEB Intrasaccular Aneurysm Treatment Device Receives FDA Approval
Jan 12, 2021
The WEB™ Aneurysm Embolization System expands with a lower profile delivery system and new size configurations.

